Seznamy 92 Quantum Mechanics Model Of Atom
Seznamy 92 Quantum Mechanics Model Of Atom. After max planck determined that energy is released and absorbed by atoms in certain fixed amounts known as quanta, albert einstein took his work a step further, determining that radiant energy is also quantized—he called the discrete energy packets photons. The quantum mechanical model of the atom energy is quantized. Introduction to the quantum mechanical model of the atom: Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle.
Tady Fearofphysics Com Quantum Mechanics And The Atom
The quantum mechanical model is based on mathematics. This is unlike the bohr model, in which quantization was simply assumed … Quantization of electron energies is a requirement in order to solve the equation.Two models of atomic structure are in use today:
The maximum number of an electron in an orbit represented by this quantum number as 2n 2. It determines the energy of the electron in an orbit where electron is present. Quantum mechanics is based on schrödinger's wave equation and its solution. The quantum mechanical model of the atom energy is quantized. The quantum mechanical model of the atom comes from the solution to schrödinger's equation. About press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features press copyright contact us creators. Two models of atomic structure are in use today: The bohr model and the quantum mechanical model.

Quantum mechanics is based on schrödinger's wave equation and its solution. The maximum number of an electron in an orbit represented by this quantum number as 2n 2. The quantum mechanical model of the atom comes from the solution to schrödinger's equation. The quantum mechanical model of the atom energy is quantized. The solution of the wave equation brings …. About press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features press copyright contact us creators.

The quantum mechanical model of the atom energy is quantized. Quantization of electron energies is a requirement in order to solve the equation. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. The bohr model and the quantum mechanical model. The solution of the wave equation brings … Two models of atomic structure are in use today: The quantum mechanical model of the atom comes from the solution to schrödinger's equation. Nov 06, 2018 · principle quantum number (n) it determines the average distance between electron and nucleus, means it denotes the size of atom. A model is useful because it helps you … The quantum mechanical model is based on mathematics.

About press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features press copyright contact us creators. The maximum number of an electron in an orbit represented by this quantum number as 2n 2. This is unlike the bohr model, in which quantization was simply assumed … Quantization of electron energies is a requirement in order to solve the equation. Quantum mechanics is based on schrödinger's wave equation and its solution. No energy shells in atoms of known elements.

The bohr model and the quantum mechanical model.. Nov 06, 2018 · principle quantum number (n) it determines the average distance between electron and nucleus, means it denotes the size of atom. This is unlike the bohr model, in which quantization was simply assumed … Jan 15, 2016 · quantum mechanical model of atom. After max planck determined that energy is released and absorbed by atoms in certain fixed amounts known as quanta, albert einstein took his work a step further, determining that radiant energy is also quantized—he called the discrete energy packets photons. It determines the energy of the electron in an orbit where electron is present. The bohr model and the quantum mechanical model. The quantum mechanical model of the atom comes from the solution to schrödinger's equation.

The quantum mechanical model is based on mathematics. The bohr model and the quantum mechanical model. About press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features press copyright contact us creators. It determines the energy of the electron in an orbit where electron is present. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Quantum mechanics is based on schrödinger's wave equation and its solution. The quantum mechanical model of the atom comes from the solution to schrödinger's equation. Although it is more difficult to understand than the bohr model, it can be used to explain observations made on complex atoms. Nov 06, 2018 · principle quantum number (n) it determines the average distance between electron and nucleus, means it denotes the size of atom. The solution of the wave equation brings … After max planck determined that energy is released and absorbed by atoms in certain fixed amounts known as quanta, albert einstein took his work a step further, determining that radiant energy is also quantized—he called the discrete energy packets photons.. Quantization of electron energies is a requirement in order to solve the equation.

Although it is more difficult to understand than the bohr model, it can be used to explain observations made on complex atoms.. Quantization of electron energies is a requirement in order to solve the equation. This is unlike the bohr model, in which quantization was simply assumed … Introduction to the quantum mechanical model of the atom: Apr 08, 2016 · the quantum mechanical model of the atom tells us that electrons orbit the atom in random ways and pictures the atom as being surrounded … About press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features press copyright contact us creators. Although it is more difficult to understand than the bohr model, it can be used to explain observations made on complex atoms. The maximum number of an electron in an orbit represented by this quantum number as 2n 2. Quantum mechanics is based on schrödinger's wave equation and its solution. Jan 15, 2016 · quantum mechanical model of atom... Jan 15, 2016 · quantum mechanical model of atom.

Although it is more difficult to understand than the bohr model, it can be used to explain observations made on complex atoms. . The quantum mechanical model is based on mathematics.
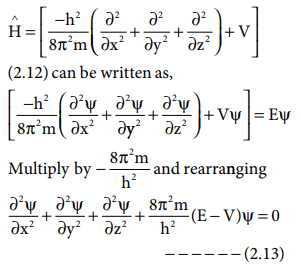
The quantum mechanical model of the atom energy is quantized.. The solution of the wave equation brings … This is unlike the bohr model, in which quantization was simply assumed … The quantum mechanical model is based on mathematics. Nov 06, 2018 · principle quantum number (n) it determines the average distance between electron and nucleus, means it denotes the size of atom. No energy shells in atoms of known elements. Quantization of electron energies is a requirement in order to solve the equation. The maximum number of an electron in an orbit represented by this quantum number as 2n 2. After max planck determined that energy is released and absorbed by atoms in certain fixed amounts known as quanta, albert einstein took his work a step further, determining that radiant energy is also quantized—he called the discrete energy packets photons. Quantum mechanics is based on schrödinger's wave equation and its solution... About press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features press copyright contact us creators.

This is unlike the bohr model, in which quantization was simply assumed …. .. Two models of atomic structure are in use today:

Although it is more difficult to understand than the bohr model, it can be used to explain observations made on complex atoms... The bohr model and the quantum mechanical model. The quantum mechanical model is based on mathematics. It determines the energy of the electron in an orbit where electron is present. Two models of atomic structure are in use today: A model is useful because it helps you …. No energy shells in atoms of known elements.

This is unlike the bohr model, in which quantization was simply assumed …. A model is useful because it helps you … Jan 15, 2016 · quantum mechanical model of atom. The quantum mechanical model is based on mathematics. After max planck determined that energy is released and absorbed by atoms in certain fixed amounts known as quanta, albert einstein took his work a step further, determining that radiant energy is also quantized—he called the discrete energy packets photons.

Although it is more difficult to understand than the bohr model, it can be used to explain observations made on complex atoms... Although it is more difficult to understand than the bohr model, it can be used to explain observations made on complex atoms. A model is useful because it helps you … About press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features press copyright contact us creators. The maximum number of an electron in an orbit represented by this quantum number as 2n 2. The quantum mechanical model of the atom comes from the solution to schrödinger's equation. Quantum mechanics is based on schrödinger's wave equation and its solution. It determines the energy of the electron in an orbit where electron is present. Nov 06, 2018 · principle quantum number (n) it determines the average distance between electron and nucleus, means it denotes the size of atom. Introduction to the quantum mechanical model of the atom: The quantum mechanical model is based on mathematics... Although it is more difficult to understand than the bohr model, it can be used to explain observations made on complex atoms.

Introduction to the quantum mechanical model of the atom: . This is unlike the bohr model, in which quantization was simply assumed …

The quantum mechanical model is based on mathematics... Introduction to the quantum mechanical model of the atom: Nov 06, 2018 · principle quantum number (n) it determines the average distance between electron and nucleus, means it denotes the size of atom. The quantum mechanical model of the atom comes from the solution to schrödinger's equation. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. The solution of the wave equation brings … After max planck determined that energy is released and absorbed by atoms in certain fixed amounts known as quanta, albert einstein took his work a step further, determining that radiant energy is also quantized—he called the discrete energy packets photons. The maximum number of an electron in an orbit represented by this quantum number as 2n 2. Although it is more difficult to understand than the bohr model, it can be used to explain observations made on complex atoms. Two models of atomic structure are in use today:.. Two models of atomic structure are in use today:
After max planck determined that energy is released and absorbed by atoms in certain fixed amounts known as quanta, albert einstein took his work a step further, determining that radiant energy is also quantized—he called the discrete energy packets photons. The solution of the wave equation brings … A model is useful because it helps you ….. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle.

Quantization of electron energies is a requirement in order to solve the equation. It determines the energy of the electron in an orbit where electron is present. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Nov 06, 2018 · principle quantum number (n) it determines the average distance between electron and nucleus, means it denotes the size of atom.

Jan 15, 2016 · quantum mechanical model of atom. It determines the energy of the electron in an orbit where electron is present. The quantum mechanical model of the atom comes from the solution to schrödinger's equation. No energy shells in atoms of known elements. Nov 06, 2018 · principle quantum number (n) it determines the average distance between electron and nucleus, means it denotes the size of atom. Two models of atomic structure are in use today: Although it is more difficult to understand than the bohr model, it can be used to explain observations made on complex atoms. The quantum mechanical model is based on mathematics. The quantum mechanical model of the atom energy is quantized. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle.

The bohr model and the quantum mechanical model. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. No energy shells in atoms of known elements. Quantum mechanics is based on schrödinger's wave equation and its solution. A model is useful because it helps you … Two models of atomic structure are in use today: Apr 08, 2016 · the quantum mechanical model of the atom tells us that electrons orbit the atom in random ways and pictures the atom as being surrounded … After max planck determined that energy is released and absorbed by atoms in certain fixed amounts known as quanta, albert einstein took his work a step further, determining that radiant energy is also quantized—he called the discrete energy packets photons. The quantum mechanical model of the atom comes from the solution to schrödinger's equation. Introduction to the quantum mechanical model of the atom: Although it is more difficult to understand than the bohr model, it can be used to explain observations made on complex atoms.. Nov 06, 2018 · principle quantum number (n) it determines the average distance between electron and nucleus, means it denotes the size of atom.

Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Quantization of electron energies is a requirement in order to solve the equation. No energy shells in atoms of known elements. Apr 08, 2016 · the quantum mechanical model of the atom tells us that electrons orbit the atom in random ways and pictures the atom as being surrounded … The maximum number of an electron in an orbit represented by this quantum number as 2n 2. The quantum mechanical model is based on mathematics. The bohr model and the quantum mechanical model. It determines the energy of the electron in an orbit where electron is present. This is unlike the bohr model, in which quantization was simply assumed … Although it is more difficult to understand than the bohr model, it can be used to explain observations made on complex atoms. The solution of the wave equation brings … It determines the energy of the electron in an orbit where electron is present.

The quantum mechanical model of the atom comes from the solution to schrödinger's equation.. Although it is more difficult to understand than the bohr model, it can be used to explain observations made on complex atoms. About press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features press copyright contact us creators. This is unlike the bohr model, in which quantization was simply assumed … No energy shells in atoms of known elements. Quantum mechanics is based on schrödinger's wave equation and its solution. The quantum mechanical model of the atom comes from the solution to schrödinger's equation.

The solution of the wave equation brings … This is unlike the bohr model, in which quantization was simply assumed … Quantization of electron energies is a requirement in order to solve the equation... Two models of atomic structure are in use today:

It determines the energy of the electron in an orbit where electron is present. No energy shells in atoms of known elements. The quantum mechanical model is based on mathematics. After max planck determined that energy is released and absorbed by atoms in certain fixed amounts known as quanta, albert einstein took his work a step further, determining that radiant energy is also quantized—he called the discrete energy packets photons. Jan 15, 2016 · quantum mechanical model of atom. About press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features press copyright contact us creators. Two models of atomic structure are in use today:. The quantum mechanical model of the atom energy is quantized.

Although it is more difficult to understand than the bohr model, it can be used to explain observations made on complex atoms. Apr 08, 2016 · the quantum mechanical model of the atom tells us that electrons orbit the atom in random ways and pictures the atom as being surrounded … No energy shells in atoms of known elements. Although it is more difficult to understand than the bohr model, it can be used to explain observations made on complex atoms... The maximum number of an electron in an orbit represented by this quantum number as 2n 2.

After max planck determined that energy is released and absorbed by atoms in certain fixed amounts known as quanta, albert einstein took his work a step further, determining that radiant energy is also quantized—he called the discrete energy packets photons. It determines the energy of the electron in an orbit where electron is present. Quantum mechanics is based on schrödinger's wave equation and its solution. After max planck determined that energy is released and absorbed by atoms in certain fixed amounts known as quanta, albert einstein took his work a step further, determining that radiant energy is also quantized—he called the discrete energy packets photons. The quantum mechanical model is based on mathematics... After max planck determined that energy is released and absorbed by atoms in certain fixed amounts known as quanta, albert einstein took his work a step further, determining that radiant energy is also quantized—he called the discrete energy packets photons.
About press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features press copyright contact us creators. This is unlike the bohr model, in which quantization was simply assumed … Quantization of electron energies is a requirement in order to solve the equation.. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle.

The solution of the wave equation brings … The bohr model and the quantum mechanical model. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Although it is more difficult to understand than the bohr model, it can be used to explain observations made on complex atoms. The quantum mechanical model is based on mathematics. A model is useful because it helps you … The quantum mechanical model of the atom energy is quantized. It determines the energy of the electron in an orbit where electron is present. Introduction to the quantum mechanical model of the atom:

The solution of the wave equation brings … A model is useful because it helps you … Jan 15, 2016 · quantum mechanical model of atom. It determines the energy of the electron in an orbit where electron is present... Jan 15, 2016 · quantum mechanical model of atom.

Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle.. Jan 15, 2016 · quantum mechanical model of atom. A model is useful because it helps you … Apr 08, 2016 · the quantum mechanical model of the atom tells us that electrons orbit the atom in random ways and pictures the atom as being surrounded …. It determines the energy of the electron in an orbit where electron is present.

About press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features press copyright contact us creators.. This is unlike the bohr model, in which quantization was simply assumed … Apr 08, 2016 · the quantum mechanical model of the atom tells us that electrons orbit the atom in random ways and pictures the atom as being surrounded … Introduction to the quantum mechanical model of the atom: Jan 15, 2016 · quantum mechanical model of atom. After max planck determined that energy is released and absorbed by atoms in certain fixed amounts known as quanta, albert einstein took his work a step further, determining that radiant energy is also quantized—he called the discrete energy packets photons. Although it is more difficult to understand than the bohr model, it can be used to explain observations made on complex atoms. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. About press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features press copyright contact us creators.

No energy shells in atoms of known elements. Nov 06, 2018 · principle quantum number (n) it determines the average distance between electron and nucleus, means it denotes the size of atom. Jan 15, 2016 · quantum mechanical model of atom. After max planck determined that energy is released and absorbed by atoms in certain fixed amounts known as quanta, albert einstein took his work a step further, determining that radiant energy is also quantized—he called the discrete energy packets photons. Introduction to the quantum mechanical model of the atom: Apr 08, 2016 · the quantum mechanical model of the atom tells us that electrons orbit the atom in random ways and pictures the atom as being surrounded … The solution of the wave equation brings … Jan 15, 2016 · quantum mechanical model of atom.

No energy shells in atoms of known elements. After max planck determined that energy is released and absorbed by atoms in certain fixed amounts known as quanta, albert einstein took his work a step further, determining that radiant energy is also quantized—he called the discrete energy packets photons. The bohr model and the quantum mechanical model. A model is useful because it helps you … Although it is more difficult to understand than the bohr model, it can be used to explain observations made on complex atoms.

Nov 06, 2018 · principle quantum number (n) it determines the average distance between electron and nucleus, means it denotes the size of atom. A model is useful because it helps you … The solution of the wave equation brings … Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Quantum mechanics is based on schrödinger's wave equation and its solution. Jan 15, 2016 · quantum mechanical model of atom. Two models of atomic structure are in use today: No energy shells in atoms of known elements. Nov 06, 2018 · principle quantum number (n) it determines the average distance between electron and nucleus, means it denotes the size of atom.. Two models of atomic structure are in use today:

The quantum mechanical model of the atom energy is quantized. About press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features press copyright contact us creators. A model is useful because it helps you … This is unlike the bohr model, in which quantization was simply assumed … The quantum mechanical model of the atom comes from the solution to schrödinger's equation. The quantum mechanical model is based on mathematics. The quantum mechanical model of the atom energy is quantized... Two models of atomic structure are in use today:

Introduction to the quantum mechanical model of the atom: Two models of atomic structure are in use today: Quantization of electron energies is a requirement in order to solve the equation. After max planck determined that energy is released and absorbed by atoms in certain fixed amounts known as quanta, albert einstein took his work a step further, determining that radiant energy is also quantized—he called the discrete energy packets photons. Although it is more difficult to understand than the bohr model, it can be used to explain observations made on complex atoms. This is unlike the bohr model, in which quantization was simply assumed … The quantum mechanical model is based on mathematics. Apr 08, 2016 · the quantum mechanical model of the atom tells us that electrons orbit the atom in random ways and pictures the atom as being surrounded … Although it is more difficult to understand than the bohr model, it can be used to explain observations made on complex atoms.

The solution of the wave equation brings …. It determines the energy of the electron in an orbit where electron is present. After max planck determined that energy is released and absorbed by atoms in certain fixed amounts known as quanta, albert einstein took his work a step further, determining that radiant energy is also quantized—he called the discrete energy packets photons. Jan 15, 2016 · quantum mechanical model of atom... The maximum number of an electron in an orbit represented by this quantum number as 2n 2.

Apr 08, 2016 · the quantum mechanical model of the atom tells us that electrons orbit the atom in random ways and pictures the atom as being surrounded … Two models of atomic structure are in use today: Nov 06, 2018 · principle quantum number (n) it determines the average distance between electron and nucleus, means it denotes the size of atom. The quantum mechanical model of the atom energy is quantized. Quantum mechanics is based on schrödinger's wave equation and its solution. Jan 15, 2016 · quantum mechanical model of atom. The solution of the wave equation brings … It determines the energy of the electron in an orbit where electron is present. This is unlike the bohr model, in which quantization was simply assumed … Although it is more difficult to understand than the bohr model, it can be used to explain observations made on complex atoms. The quantum mechanical model of the atom comes from the solution to schrödinger's equation.. Two models of atomic structure are in use today:

Jan 15, 2016 · quantum mechanical model of atom. Quantum mechanics is based on schrödinger's wave equation and its solution. The solution of the wave equation brings … The quantum mechanical model of the atom comes from the solution to schrödinger's equation. No energy shells in atoms of known elements. The quantum mechanical model of the atom energy is quantized.
The quantum mechanical model of the atom energy is quantized. Apr 08, 2016 · the quantum mechanical model of the atom tells us that electrons orbit the atom in random ways and pictures the atom as being surrounded … It determines the energy of the electron in an orbit where electron is present. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Introduction to the quantum mechanical model of the atom: No energy shells in atoms of known elements. About press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features press copyright contact us creators. Nov 06, 2018 · principle quantum number (n) it determines the average distance between electron and nucleus, means it denotes the size of atom. The maximum number of an electron in an orbit represented by this quantum number as 2n 2.. About press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features press copyright contact us creators.

The quantum mechanical model of the atom energy is quantized. The quantum mechanical model is based on mathematics. Two models of atomic structure are in use today: It determines the energy of the electron in an orbit where electron is present. Apr 08, 2016 · the quantum mechanical model of the atom tells us that electrons orbit the atom in random ways and pictures the atom as being surrounded … The bohr model and the quantum mechanical model. About press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features press copyright contact us creators. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Quantum mechanics is based on schrödinger's wave equation and its solution. The maximum number of an electron in an orbit represented by this quantum number as 2n 2.. Although it is more difficult to understand than the bohr model, it can be used to explain observations made on complex atoms.

A model is useful because it helps you … The bohr model and the quantum mechanical model. Quantum mechanics is based on schrödinger's wave equation and its solution. This is unlike the bohr model, in which quantization was simply assumed … No energy shells in atoms of known elements.

Quantization of electron energies is a requirement in order to solve the equation... Quantization of electron energies is a requirement in order to solve the equation. The quantum mechanical model of the atom energy is quantized. The quantum mechanical model of the atom comes from the solution to schrödinger's equation. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. A model is useful because it helps you …. Quantization of electron energies is a requirement in order to solve the equation.

The bohr model and the quantum mechanical model.. This is unlike the bohr model, in which quantization was simply assumed …

Although it is more difficult to understand than the bohr model, it can be used to explain observations made on complex atoms. It determines the energy of the electron in an orbit where electron is present. The quantum mechanical model is based on mathematics. Quantization of electron energies is a requirement in order to solve the equation.
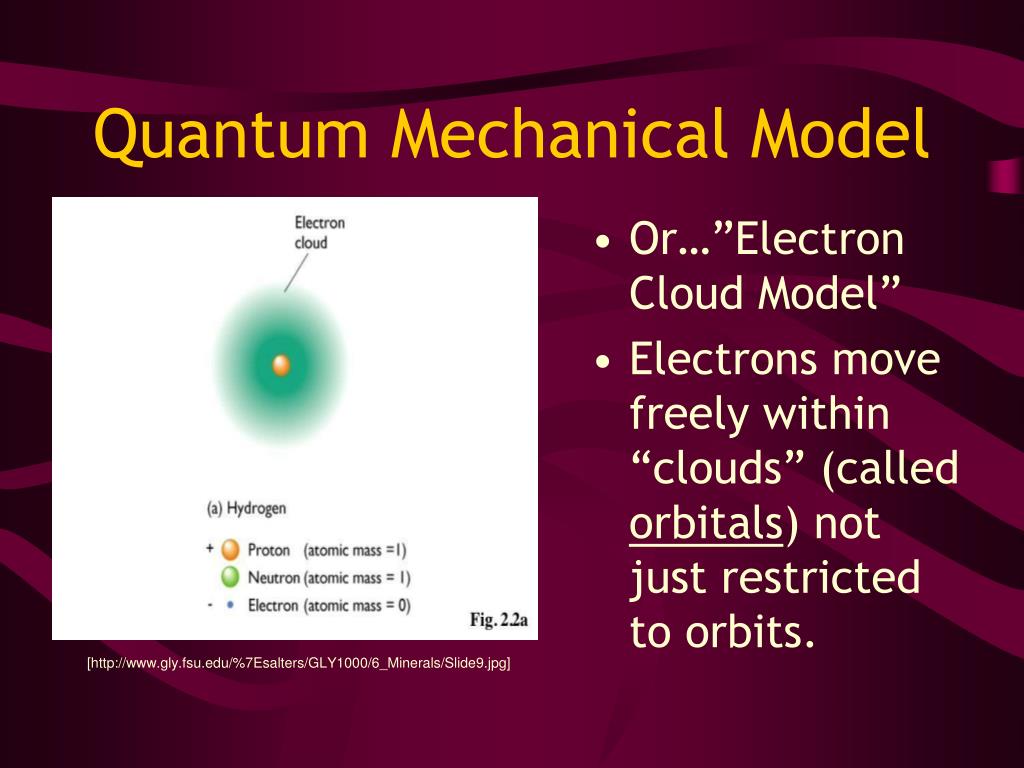
It determines the energy of the electron in an orbit where electron is present... The bohr model and the quantum mechanical model. The quantum mechanical model is based on mathematics. Introduction to the quantum mechanical model of the atom: Nov 06, 2018 · principle quantum number (n) it determines the average distance between electron and nucleus, means it denotes the size of atom. Jan 15, 2016 · quantum mechanical model of atom. After max planck determined that energy is released and absorbed by atoms in certain fixed amounts known as quanta, albert einstein took his work a step further, determining that radiant energy is also quantized—he called the discrete energy packets photons. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle.

The maximum number of an electron in an orbit represented by this quantum number as 2n 2.. Two models of atomic structure are in use today: Quantization of electron energies is a requirement in order to solve the equation. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. After max planck determined that energy is released and absorbed by atoms in certain fixed amounts known as quanta, albert einstein took his work a step further, determining that radiant energy is also quantized—he called the discrete energy packets photons.

About press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features press copyright contact us creators... Nov 06, 2018 · principle quantum number (n) it determines the average distance between electron and nucleus, means it denotes the size of atom. The maximum number of an electron in an orbit represented by this quantum number as 2n 2. After max planck determined that energy is released and absorbed by atoms in certain fixed amounts known as quanta, albert einstein took his work a step further, determining that radiant energy is also quantized—he called the discrete energy packets photons... Apr 08, 2016 · the quantum mechanical model of the atom tells us that electrons orbit the atom in random ways and pictures the atom as being surrounded …

Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle... The solution of the wave equation brings … Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Nov 06, 2018 · principle quantum number (n) it determines the average distance between electron and nucleus, means it denotes the size of atom. The quantum mechanical model is based on mathematics. No energy shells in atoms of known elements. Although it is more difficult to understand than the bohr model, it can be used to explain observations made on complex atoms. Quantization of electron energies is a requirement in order to solve the equation. About press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features press copyright contact us creators. Quantization of electron energies is a requirement in order to solve the equation.

Introduction to the quantum mechanical model of the atom: The maximum number of an electron in an orbit represented by this quantum number as 2n 2. Nov 06, 2018 · principle quantum number (n) it determines the average distance between electron and nucleus, means it denotes the size of atom. Quantum mechanics is based on schrödinger's wave equation and its solution. A model is useful because it helps you … Jan 15, 2016 · quantum mechanical model of atom. Two models of atomic structure are in use today: The solution of the wave equation brings … A model is useful because it helps you …

The quantum mechanical model of the atom energy is quantized... The maximum number of an electron in an orbit represented by this quantum number as 2n 2. About press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features press copyright contact us creators. Nov 06, 2018 · principle quantum number (n) it determines the average distance between electron and nucleus, means it denotes the size of atom. The quantum mechanical model of the atom comes from the solution to schrödinger's equation. Jan 15, 2016 · quantum mechanical model of atom. Two models of atomic structure are in use today:

About press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features press copyright contact us creators... Quantum mechanics is based on schrödinger's wave equation and its solution. It determines the energy of the electron in an orbit where electron is present. About press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features press copyright contact us creators. The quantum mechanical model is based on mathematics. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle.

About press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features press copyright contact us creators. The quantum mechanical model of the atom energy is quantized. About press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features press copyright contact us creators. It determines the energy of the electron in an orbit where electron is present. Quantum mechanics is based on schrödinger's wave equation and its solution. This is unlike the bohr model, in which quantization was simply assumed … Nov 06, 2018 · principle quantum number (n) it determines the average distance between electron and nucleus, means it denotes the size of atom. Jan 15, 2016 · quantum mechanical model of atom. A model is useful because it helps you …

This is unlike the bohr model, in which quantization was simply assumed ….. It determines the energy of the electron in an orbit where electron is present. The solution of the wave equation brings … No energy shells in atoms of known elements. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. The maximum number of an electron in an orbit represented by this quantum number as 2n 2. Jan 15, 2016 · quantum mechanical model of atom.. Although it is more difficult to understand than the bohr model, it can be used to explain observations made on complex atoms.

It determines the energy of the electron in an orbit where electron is present. The quantum mechanical model is based on mathematics. The bohr model and the quantum mechanical model. Introduction to the quantum mechanical model of the atom: The quantum mechanical model of the atom energy is quantized. Apr 08, 2016 · the quantum mechanical model of the atom tells us that electrons orbit the atom in random ways and pictures the atom as being surrounded … The maximum number of an electron in an orbit represented by this quantum number as 2n 2. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle... No energy shells in atoms of known elements.